
Part of Brands:



Part of Brands:


If you missed any of our presentations from our Academy Studio, we have collected them below.
The way we perform ICSI have been the same for many years, placing the oocytes most of the time in a buffer medium to avoid pH changes. In this talk Dr. Robert Mendola, Lab Director, CCRM-NJ, USA, looks at the effects of HEPES and MOPS buffered medium and it’s impact on the oocytes when compared with doing the ICSI procedure in a bicarbon buffered medium.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Hear 2 experts in the field, Prof. Thomas Freour, Head of infertility department & ART centre, University Hospital of Nantes, France & Dr. Juergen Liebermann, Director of Laboratories, Fertility Centres of Illinois, USA, talk about their research and clinical experience with the use of an ultra fast warming protocol and the practical benefits it has inside the laboratory.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Elena Linara-Demakakou, Head of Embryology, from the London Woman’s Clinic in the UK shares her experience of using the iDAScore embryo evaluation software with the Gx media in their clinic.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
In this panel discussion with Dr. Rita Vassena, CEO Fecundis, Spain, Dr. Mina Popovic, Group Scientific Director, Eugingroup, Spain & Dr. Denny Sakkas, CSO, Boston IVF, IVIRMA, USA, they share their thoughts on what we can expect about the future of IVF with regards to possible changes in technology, the culture environment and the way procedures are being performed.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Listen how Dr. Leyre Cordero, Embryologist, from the Ovobank Group in Spain explains how they use a range of different genetic tests to ensure they screen their oocyte donors thoroughly and limit the possibility of genetically abnormal gametes being used in their program.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Advances in genetic testing technology allows for a more patient friendly experience. Listen how Dr. Miguel Milan, Prenatal Genetic Diagnosis Director, Vitrolife Group, Spain, explains the use and application of a non-invasive test for the products of conception.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
In this very interesting presentation, Dr. Maria Escribá, Embryologist, Equipo Juana Crespo, Spain, demonstrates that with the proper genetic testing of 3PN embryos, one can save genetically normal embryos from being discarded.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Following a multi-centre RCT on the use of iDAScore as an embryo selection tool Dr. Peter Illingworth, Medical Director, Virtus Health, Australia, explains how the use of this system can save time 10 fold during embryo evaluation in the laboratory.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
In the light of recent recalls of embryo culture media and oil, Dr. Nuno Costa-Borges, Scientific Director, Co-Founder, Embryotools, Spain & Dr. Mark Larman, Vice President, Academy, Vitrolife Group, Sweden, share their thoughts on the testing of embryo culture media and how an appropriate Mouse Embryo Assay is still our best tool for identifying sub-optimal and toxic components.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Listen how Dr. Khaled Pocate, Senior Embryologist, and his team from Cochin-Port Royal University Hospital in France make use of the Octax Laser system for their day-to-day clinical work as well as ongoing research projects.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Dr. Kelly Tilleman, IVF Laboratory Director, UZ Gent, Belgium, demonstrates the implementation and use of an electronic witnessing system in their IVF laboratory to ensure the safe tracking and identification of their patients’ gametes and embryos at all times during the IVF process.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Dr. Cristina Pastor-Leary, Embryologist, Kinderwunch Institut, Austria, shares her positive experience of using a non-invasive method for the genetic testing of embryos in their PGT-A program.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Non-humidified incubation of embryos is not the same as dry incubation. Listen how Francesca Bahr, Science and Technology Analyst, Vitrolife Group, Denmark, explains the difference and demonstrates that the Embryo Scope Time-lapse system is a safe culture environment for embryos through its advanced technological design.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
In this panel discussion moderated by Dr. Laura Girardi i, we get insights from Dr. Montse Duran, Fertility Consultant, The Centre for Reproductive & Genetic Health, UK, & Dr. Manuel Fernandez, Medical Director, VIDA Seville, Spain, and how strict and proper protocol application on their patients allow them to use this endometrial test to secure optimal outcomes for their patients.
This presentation was part of Vitrolife Group live Academy Studios from ESHRE 2024.
Earlier this year, the Vitrolife Group acquired eFertility an innovative system and software company transforming IVF clinic management with cutting-edge solutions.
This acquisition is a key element of the Vitrolife Group’s strategy to bring increased standardisation and digitalisation to IVF clinics around the world. Stop by our booth at ESHRE for a demonstration and to learn more about how our newest addition, eWitness, can optimise your lab workflow and enhance security with reliable insights. You can also visit eFertility’s booth in Hall 11 (11.077).
Read the press release here
The Vitrolife Group has a vision with a purpose: to enable people to fulfill the dream of having a healthy baby. Together, we combine high-quality medical devices with pioneering genetic testing solutions, covering the entire reproductive-health journey. Grounded in science, our innovations provide consistent performance, workflow efficiency, and guaranteed quality. Together, we create excellence in reproductive health!
Discover our values through the voices of Vitrolife Group employees
A new enhanced version of our PGT-A test that now also includes features such as Genetic PN check, contamination and sibling quality control.
Based on more than 70.000 samples we can now detect bacteria at the species level, allowing for more targeted treatments.

A non-invasive, tissue-independent approach for assessing the origin of early pregnancy loss, determining chromosomal abnormalities, through a simple blood test.
Endometrial Receptivity Analysis
Maximize the chances of pregnancy without losing valuable embryos

Carrier Genetic Test
Determines the risk of having a child with a genetic disease

iDAScore® is an AI-based scoring system that provides fully automated analysis of time-lapse sequences.
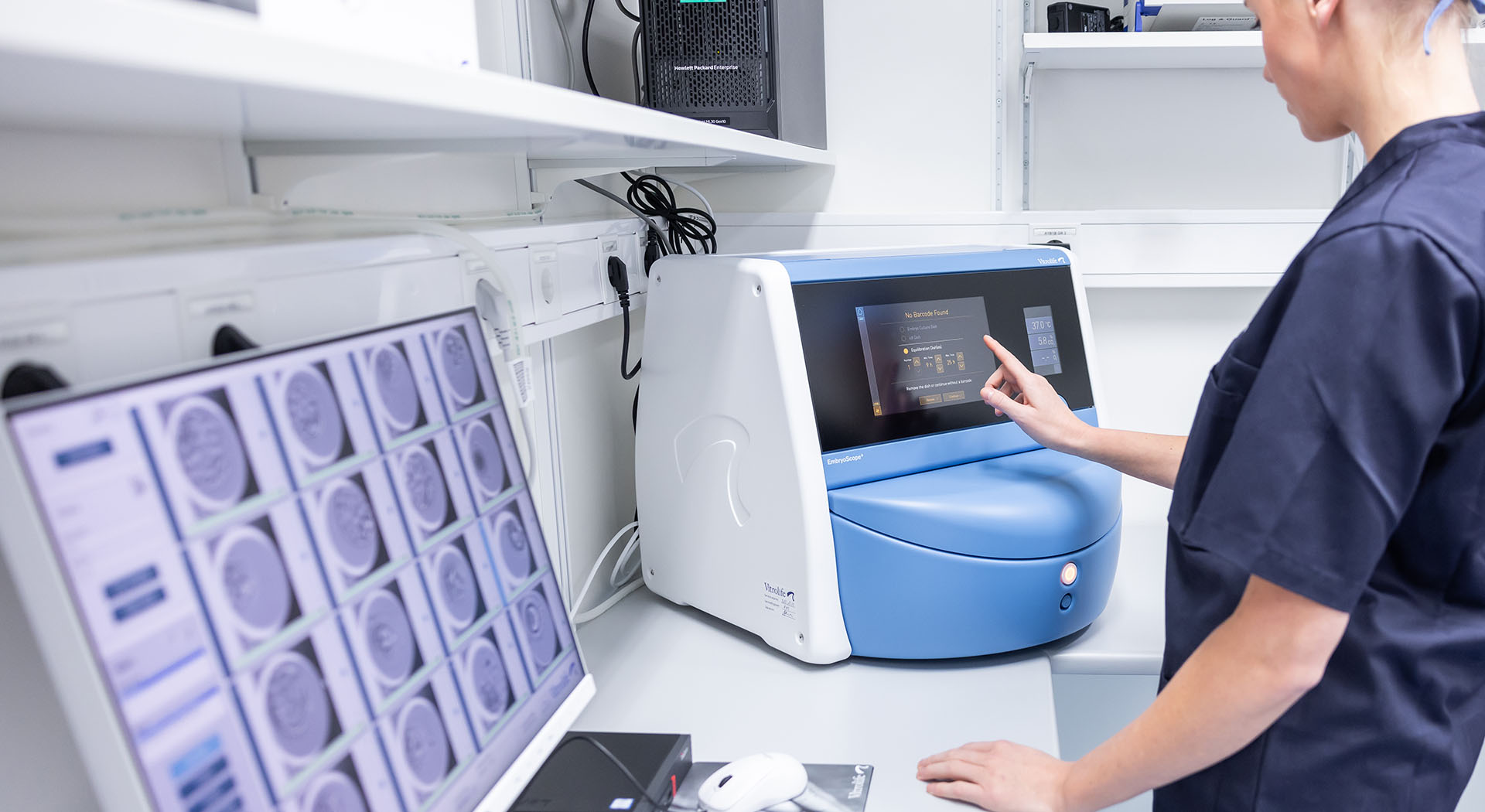
EmbryoScope+ is an incubator that incorporates time-lapse technology, providing a stable culture environment for embryos. Time-lapse technology reduces observational time restrictions and stress to embryos during culture and assessment.

Gx Media is our latest addition in our media portfolio that includes triple antioxidant protection for improved embryo viability.
EmbryoGlue® is an implantation medium developed exclusively for transfer, and is the only existing transfer medium with a proven implantation-enhancing effect.
OVOIL™ is a 100% paraffin oil. OVOIL HEAVY™ is a synthetically high viscosity oil. Both of them are produced in a highly controlled process from extensively tested and selected LOTs of raw material.